| CAS
NO. |
13463-41-7 |
|
| EINECS
NO. |
236-671-3 |
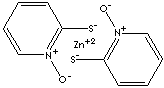
| FORMULA |
C10H8N2O2S2Zn |
| MOL
WT. |
317.68 |
|
H.S.
CODE
|
2933.39 |
|
TOXICITY
|
|
| SYNONYMS |
Zinc
bis-(2-Pyridinethiol-1-oxide); ZNPT; ZPT; |
| Zinc,
bis(2-pyridylthio)-, N,N'-dioxide; 1-Hydroxy-2-pyridinethione,
zinc salt; 2(1H)-Pyridinethione, 1-hydroxy-, zinc complex;
2-Mercaptopyridine 1-oxide Zinc Salt; 2-Pyridinethiol
N-oxide zinc salt; 2-Pyridinethiol, 1-oxide, zinc salt;
Zinc,
Bis(2-pyridinylthio)-, N,N'-dioxide; Zinc, Bis(2-pyridylthio)-,
1,1'-dioxide; Zinc, Bis(2-pyridylthio)-, N,N'-dioxide;
Zincpolyanemine; Zinksalz Des 1-hydroxi-2-pyridinthion; |
| SMILES |
|
|
CLASSIFICATION
|
DISINFECTANTS
/
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
Off-white
to tan powder
|
| MELTING
POINT |
>
240 C (Decomposes) |
| BOILING
POINT |
|
| SPECIFIC
GRAVITY |
|
| SOLUBILITY
IN WATER |
Insoluble |
| pH |
6.0
- 8.0 |
| VAPOR
DENSITY |
|
| AUTOIGNITION |
|
|
REFRACTIVE
INDEX
|
|
| NFPA
RATINGS |
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions |
|
APPLICATIONS
|
|
Zinc
Pyrithione inhibits the growth of fungi, yeast, mold
and bacteria. It is used in as an active ingredient
in cosmetics. It is used as an anti-dandruff
ingredient in shampoo preparations. It is used in used
in antifouling paints and sealants. Amine N-oxides are active components in body care products such as shampoo,
bubble bath, and hand-soap formulations as they are cationic and can act as a mild conditioner
in acidic media.
In neutral or weak basic media, they are featured as excellent stabilizer
and viscosity building provider.
Pyrithione
N-oxide molecules
|
Product
|
CAS
RN
|
| Pyrithione |
1121-30-8 |
2-Mercaptopyridine N-oxide
|
1121-31-9
|
| Pyrithione zinc |
13463-41-7 |
|
154592-20-8 |
| Pyrithione sodium |
15922-78-8;
3811-73-2 |
| Dipyrithione |
3696-28-4 |
| Bispyrithione magsulfex |
67182-81-4 |
|
| SALES
SPECIFICATION |
|
POWDER
|
|
APPEARANCE
|
Off-white
to tan powder
|
| ASSAY |
95.0%
min
|
|
pH
|
6.0
- 8.0
|
|
AQ.
DISPERSION
|
|
APPEARANCE
|
clear
liquid
|
| ACTIVE
MATTER |
48.0%
min
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD
CLASS |
6.1
(Packing Group: III) |
| UN
NO. |
2811 |
| OTHER
INFORMATION |
|
Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases:
24/25
|
|
GENERAL DESCRIPTION
OF ZINC MINERAL |
|
Zinc is an essential mineral having a role in the maintenance of the body's
nervous and immune systems (T-cell function). This mineral is involved in the biochemical
reactions as an antioxidant in the healing process and develops normal tissues
Zinc is a cofactor in enzymatic reactions such as protein synthesis polymerases
and in carbonic acid anhydrase. Zinc maintains the body's alkaline balance. Zinc
finger, a structural domain found in many gene-regulatory proteins, is a
component of hydrophobic hormones acting stabilizing the biomembrane structures
and cell membrane metabolism. Zinc deficiencies may result in prolonged wound
healing, delayed sexual maturation, mental lethargy, skin changes, and
susceptibility to infections. Gluconate and citrate forms are mainly used as
zinc supplements. They are easily absorbed by the body. |
|
PRICE |
